Abstract
Results of numerous experimental studies indicate that low-intensity ultrasound (US) intensifies the drug induced fibrinolysis. However, exits opinion, what high-intensity ultrasound could be not recommending for use in combination with thrombolytic agents due to the transience of exposure of thrombus to ultrasound and relatively low speed of the enzymatic process. We also hypothesise that high-intensity low-frequency US can accelerate the drug induced fibrinolysis. During in vitro studies, we showed that the administration of streptokinase (SK) prevents the formation of particle conglomerates resulting due to destruction of fibrin clots by using low-frequency high-intensity US. In addition, it was established that in conditions of the combined effect of streptokinase and high-intensity US, the process of acoustic-mechanical destruction of thrombus is prevailing, and the fibrinolytic effect of streptokinase is generally witnessed after the termination of US and manifests itself in enzymatic lysis of both resultant particles and residual clot mass. In this study we verified the effect of low-frequency high-intensity ultrasound on the dynamics and efficiency of fibrinolysis induced by streptokinase on various structure thrombi model, characteristic features of the fibrin clot network structure occurring during the combined effect of ultrasound and streptokinase.
1. Material and methods
In our study we used ultrasound system (US) consisting of the ultrasound generator, piezoelectric transducer and concentrator waveguide was used in the study. Generator with the output of 80 W was equipped with an automatic frequency control loop for resonance. Smooth frequency variation within the range of 32–45 kHz allowed setting the generator for the resonance with acoustic system. US generator operates in batch mode with 50 % cycle occupancy, which duration of 1 sec. US intensity amounted to 30 W/cm², and this corresponded to the variation of amplitude of longitudinal oscillation of the distal end of a waveguide of 85 μm. The specific power of US energy at the operating end of a waveguide was established by using the calorimetric method presented in the study [9].
Methodology of Fibrin Clots and Clots of Whole Blood Formation. Fresh citrated donor blood obtained from the Republican Blood Transfusion Station of the Ministry of Health of the Republic of Belarus was used for the study. Blood serum was obtained by centrifugation of blood at 2000 g for 10 min. Fibrin clots were created from blood serum, 1000 mcL of which was mixed with 20 mcL of thrombin solution with an activity of 50 NIH, after subsequent incubation. Clots of whole blood were formed in the same manner from donor blood diluted by its own plasma in advance, until hematocrit of 20 % was achieved. Thrombin (50 NIH) was added to 5 ml of donor blood in the ratio 9:1, and incubating at room temperature for 2 or 24 hours. Furthermore, fresh blood clots were used.
Thrombosis Model. As soon as thrombin was added to blood serum, 500 kcL of obtained mixture was carefully, in order to prevent foam formation, moved inside the horizontal polyvinylchloride transparent tube with a length of 30 cm and inner diameter of 3.5 cm, distal end of which was clipped in advance. Inner surface of the tube was coarse, and this allowed ensuring good fixation of a clot. After the 30-minute incubation and development of 2 cm clot, horizontal tube was bent to give it like a U-shape. The tube was filled with isotonic solution of NaCl from the distal end, and from and isotonic solution of NaCl, or mix with SK solutionform the proximal end, depending on the experiment conditions. Variation of the size of fluid column above the proximal end of the clot allowed obtaining various values of pressure gradient. Maximum value of pressure gradient whereby the clot did not detach from the walls of plastic tube was 8 cm of the water column.
2. Experimental methodology
Destruction of the model thrombus formed in a polyvinylchloride tube in the conditions of excessive pressure gradient created by physical solution or SK was performed using the following methods:
1. Exposure to low-frequency US at an intensity of 11.2; 21.6; 30.2; 42.4; 54.2 W/cm2 for 1, 2, 3, 4 and 10 minutes in physiological solution. Waveguide was inserted into the standard 7F catheter 7F upon adjusting its radiant head at a distance of 2–3 mm from the proximal end of the clot. Exposure to US was carried out under visual control, while moving the waveguide from the proximal end of fibrin/blood clot to the distal end, and vice versa.
2. Administration of 500 units/ml dose of streptokinase (SK) into 2 ml of physiological solution for 10 minutes under a pressure of 5 cm water column with the subsequent incubation (without the pressure) over a period of 30 minutes. Presented values of dose, time of exposure and level of pressure gradient of SK solution on fibrin/blood clots in preliminary experiments contributed to achieve the maximum percent of their lysis. This qualified this scheme of enzymatic thrombolysis as an optimal one.
3. Administration of SK for 10 minutes under a pressure of 5 cm water column with the exposure to low-frequency US at an intensity of 11.2; 21.6; 30.2; 42.4; 54.2 W/cm2 for 3 minutes (SK dose and administration method were identical to those in the previous thrombi destruction method). After the exposure to US, incubation of residual clot was performed (without the pressure) over a period of 30 minutes.
During the whole period of blood serum exposure to sound, the temperature control was carried out using electrical contact thermometer TPS-1 (Russia). It was established in advance that the effect of US at an intensity of 11.2–54.2 W/cm2 for 5 minutes in a test tube enables the temperature to rise from 5 to 10 °С. In relation to this, thermostat control of studied samples was carried out during the whole period of experimental study. As a result, the difference in the temperature of solutions of studied samples until and after the exposure to sound amounted to 0.2 °С.
The efficiency of various methods of thrombi destruction was evaluated according to the reduction of clot mass ) which was calculated by using the following formula:
where is initial clot mass, is residual clot mass.
In cases of fibrin clot formation, the residual fibrin registered by using the spectrophotometric method [10] and was considered as the residual mass. Part of the clot remaining after the exposure was retrieved from the tube, pressed out on the glass from the corresponding environment of occupancy of model system, and then three times washed three times in the isotonic NaCl solution. Subsequently, the clot was transferred into a special dish containing 1.0 ml 1N solution of NaOH, which was then slowly heated up at the temperature of 60 °С for 30 minutes. After the full dissolution of fibrin, solution extinction at 560 nm was registered on the spectrophotometer Beckman DU-640 (Germany). The difference of extinction value registered at 560 nm and multiplied by the conversion factor for the calculation of conditional fibrinogen concentration corresponding to the residual clot fibrin. The content of fibrin in clot was expressed in relative units (%) what takes possibility to compare obtained results.
With blood clot, the evaluation of its residual mass was performed. In order to do this, parts of blood clot that were not destroyed were thoroughly washed with distilled water, and then transferred into Petri dishes and placed to the thermostat for 24 hours at the temperature of 37 °С in order to remove moisture. After 24 hours, dry clot residual was weighted on electronic scales and its mass was measured.
Scanning electron microscopy was performed during the destruction of fibrin clots by using of three methods:
Exposure to US at an intensity of 21.6 and 30.2 W/cm2 for 3 minutes;
Exposure to 500 units/ml dose of SK under pressure of 5 cm water column for 10 minutes with the subsequent incubation (in norm tension conditions) for 30 minutes;
Exposure to 500 units/ml dose of SK under pressure of 5 cm water column for 10 minutes with the subsequent exposure to US at an intensity of 21.6 and 30.2 W/cm2 for 3 minutes and incubation in SK environment (in norm tension conditions) for 30 minutes.
Residual clots obtained after the effect of model thrombus exposure to three methods were washed thoroughly with the distilled water and subsequently dried at a room temperature.
The study of fibrin clot structure parameters was carried out using Autoscan image analysis software system developed by ZAO Spektroskopicheskiye Sistemy (Spectroscopic Systems) and Sevchenko Research Institute of Applied Physical Problems of the Belarusian State University, with the participation of Research Institute of Powder Metallurgy.
3. Results
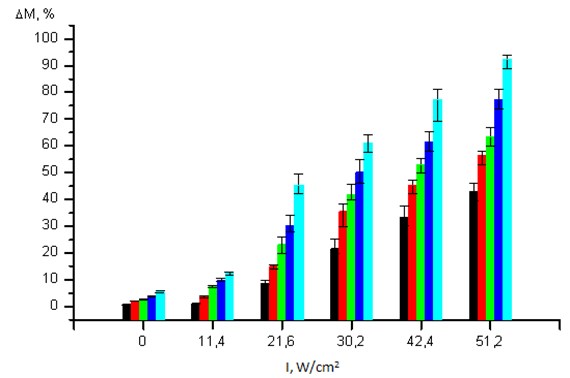
An Efficiency of Thrombi Destruction Using US. Direct correlation was established between the efficiency of destruction of fibrin clots and the intensity and duration of exposure to US. Exposure to US at an intensity of 11.2 W/cm2 for 1 minute does not ensure the positive destruction of a clot in comparison with the initial value and mechanical impact ( 0.05) (Fig. 1). When US intensity is increased to 51.2 W/cm2 and the duration increased to 10 minutes, the clot destruction degree amounts to 92.50 (89.00; 94.00) %.
In order to evaluate the specific contribution of each indicated US parameter, we carried out a multivariate analysis. It was established that US intensity has the greatest effect on reduction of clot mass (correlation score of 0.93), and its duration has the lowest effect (correlation score of 0.31).
Considering the lowest effect of duration on the destruction degree, all further experiments were performed with one time of exposure to sound but various values of US intensity. The time of exposure to US was determined according to minimal intensity level, at which the reduction of clot mass could be sufficient for occurring of reperfusion of affected organs and systems. The effect of US at an intensity of 21.6 W/cm2 or higher for 3 minutes causes > 20 % reduction of clot mass, and this is sufficient for the occurring of reperfusion. Accordingly, the duration of US had impact on clots in all further experiments amounted to 3 minutes.
Unlike the mechanical impact, exposure to US contributes to significant destruction of blood clot, which degree directly correlated with intensity. Comparative evaluation of US impact on fibrin and fresh blood clots shows that the latter is destroyed positively better in the same conditions ( 0.01). In particular, at an intensity of 21.6 W/cm2 the percentage of destruction of blood clot reached 67.44 (61.23; 70.74) %, at 30.2 W/cm2 it was 87.85 (84.19; 91.20) %, and at 51.2 W/cm2 it was 94.29 (92.11; 98.32) %. At the same time, the percentage of destruction of fibrin clot upon its exposure to US of the same intensity amounted to 23.25 (19.70; 26.00) %, 53.05 (50.10; 55.40) % and 63.50 (60.00; 67.00) %.
Fig. 1Dependence of fibrin clot mass reduction values (ΔM, %) on the intensity of exposure to US (I, W/cm2) for a duration of 1 min (), 2 min (), 3 min (), 4 min () and 10 min (). Value of 0 intensity corresponds to mechanical impact
Effect of Streptokinase on the Efficiency of Thrombi Destruction By Using Ultrasound. Results of our study confirm the significance of effect of pressure gradient on the efficiency of drug induced fibrinolysis. In preliminary experiments it was shown that at the incubation of fibrin clot with 500 units/ml dose of SK for 60 minutes the clot mass reduction amounted to 2.65 (2.33; 2.90) %. At the same time, administration of SK into a fibrin clot over the period of 10 minutes under a pressure of 5 cm of water column contributed to its significant destruction: in 40 minutes clot mass was reduced for 43.50 (39.50; 46.50) %. Additional mechanical impact after the administration of SK did not have substantial effect on the enzymatic lysis of a clot. On the contrary, exposure to US contributed to statistically significant clot mass reduction in comparison to the effect of SK. alone. With other equal conditions, the final reduction of fibrin clot mass was depended from the US intensity. Accordingly, the maximum degree of clot destruction was witnessed upon the US impact at an intensity of 51.2 W/cm2. It amounted to 73.12 (71.00; 78.00) and 79.64 (75.00; 82.50) %.
In the conditions of preliminary administration of SK into the clot, exposure to US contributed to the significant destruction of a blood clot as well. However, in this case the final clot mass reduction, on the one side, was determined by the intensity of exposure to US, and from the other side, by the degree of clot retraction. At the same time, in other equal conditions, the final mass reduction of a fresh 2-hour clot was positively different from the reduction of a 24-hour clot ( 0.01). However, as soon as they were exposed to US of various intensities, the positive difference in mass reduction of clots with different retraction degree was not revealed. For example, immediately after the impact of US of 21.6 W/cm2, fresh clot mass was reduced by 67.00 (64.00; 74.50) %, and 2- and 24-hour clot mass by 68.20 (62.00; 72.00) % and 64.65 (62.00; 67.00) % accordingly.
Scanning Electron Microscopy of Fibrin Clots. After the exposure to SK fibrin tissues oriented at the initial state mostly in one direction. Image quantitative analysis demonstrated that the length and diameter of fibrin tissue have positively reduced in comparison to the control. At the same time, the insignificant increase of pore size from 0.26 ± 0.08 μm to 0.31 ± 0.09 μm ( 0.05) was established.
In conditions of administration of SK in combination with US at intensity of 21.6 W/cm2 and 30.2 W/cm2, numerous irregular tissue fractures were witnessing as well. Porosity of fibrin structure increased significantly. Quantitative analysis of network parameters carried out in those conditions showed the significant decrease of fibrin tissue sizes in comparison to the control ( 0.01). In addition, the degree of tissue size reduction depended on US intensity. At an intensity of 21.6 W/cm2,tissue length was reduced to 2.09 (1.96; 2.23) μm, and at an intensity of 30.2 W/cm 2, it wasreduced to 1.62 (1.40; 1.95) μm, ( 0.05). Tissue diameter was changing analogically. Furthermore, effect of SK in combination with US contributed to the significant increase of pore sizes from 0.26 ± 0.08 μm in control to 0.35 ± 0.09 μm and 0.37 ± 0.08 μm accordingly at the US intensities of 21.6 W/cm2 and 30.2 W/cm2 (Table 1).
Table 1Main parameters of fibrin clot structure after various thrombi destruction methods
Fibrin clot parameters | Groups | ||||
Initial value | SK | US, 30.2 W/cm2 | SK+US, 21.6 W/cm2 | SK+US, 30.2 W/cm2 | |
Fibrin tissue length (n = 20) | 5.22 (4.87; 5.94) | 5.20 (4.87; 5.59) | 2.50 (2.13; 2.94) * | 2.09 (1.96; 2.23) * | 1.66 (1.40;1.95)* |
Fibrin tissue width (n = 51) | 0.20 (0.17; 0.23) | 0.18 (0.16; 0.22) | 0.15 (0.13; 0.16) *† | 0.13 (0.11;0.15) * | 0.11 (0.09;0.13)* |
Pore size (n = 115) | 0.26 ± 0.08 | 0.29 ± 0.09 | 0.31 ± 0.09 | 0.35 ± 0.09* | 0.37 ± 0.08* |
Note: SK means streptokinase, US means ultrasound. * - 0.01 in comparison to the initial value, † - 0.05 in comparison to the analogous value after the exposure to SK | |||||
4. Discussion
Results of our in vitro study show that with the use of SK, the significant increase of efficiency of various structure model thrombi exposure to high-intensity US was witnessed; the efficiency was evaluated according to their mass reduction degree. In consideration of the aforementioned, it is possible to make a conclusion that upon the impact of high-intensity US, the acceleration of medicamentous fibrinolysis was observed.
It was established that when other conditions were equal, exposure to US at various intensities caused more significant destruction of blood clots in comparison to fibrin clots. This shows their higher response to such interventions. Noted differences in the degree of US destruction can be related to lower immunity of blood clots to mechanical impact due to the presence of erythrocytes. It is also known that the presence of erythrocytes in fibrin network has a negative impact on in vitro fibrin polymerization [17]. As a result, pores of significant size (up to 5 μm in average) occur in whole blood clots as compared to fibrin clots, and they certainly have negative impact on clot immunity to external action. On the other hand, exposure of a blood clot to US causes the destruction of its cellular elements, and this contributes to the formation of natural lumens [18]. In general, those processes may potentiate the cavitation flow and decrease cavitations threshold, and this is the basis of high efficiency of exposure of blood clots to US.
Presence of SK has a significant impact on acoustic-induced changes of fibrin clot structure, which manifests itself in further decrease of the length and cross dimension of fibrin tissue, as well as increase of pore size. It is possible to assume that the basis of noted changes is the disintegration of fibrin tissue upon exposure to US, which contributes to the increase of streptokinase penetration into the clot, and, due to the increase of additional sites for plasminogen/plasmin binding with fibrin, intensifies its proteolytic breakdown. Results of electron microscopy obtained by us showing destruction of fibrin tissues mediated by plasmin which occurs due to lysis front movement on the outside of tissue to the inside and the tissue diameter reduction [19]. The basis of revealed consistent are confirmed by the data found in literature showing that the destruction of fibrin tissues mediated by plasmin occurs due to lysis front movement on the outside of tissue to the inside, and the tissue diameter is reduced at that [19]. The basis of revealed consistent patterns is the whole range of complex physical-chemical phenomena, which are resulting from the exposure to US. At that, the cavitation and acoustic microflows are the main operating mechanisms of thrombi destruction by using US [20, 21, 22]. The formation of cavitation bubble in the liquid medium and its subsequent rupture in corresponding conditions is accompanied by the formation of impact wave, which causes the change of fibrin clot structure, clearly established in our study. Moreover, upon the expansion of a powerful acoustic field in the fluid, as result occurs a usually vertical acoustic microflows [23] which increase diffusion processes between heterogeneous phases [20]. Many studies show that particles of fluid medium start oscillating with a certain speed upon the US impact [20, 22, 24]. For example, at an intensity of 10 W/cm², the particle speed reaches 10 cm/sec [25]. As acoustic microflows occur, particle speed increases even more. Accordingly, decondensation and mollities of thrombus surface is witnessed in this fast medium “microintermixing”, and this also may have a positive effect on diffusion of clot-busting drugs inside a clot along with the cavitation.
Results of our in vitro study shows that use of SK significantly increases efficiency of various model thrombi exposure to high-intensity US and this efficiency was evaluated according to their mass reduction degree. In consideration of the aforementioned, it is possible to make a conclusion that upon the impact of high-intensity US, the acceleration of drug induced fibrinolysis was observed.
5. Conclusions
The presence of streptokinase has significant impact on acoustic-induced changes of fibrin clot structure: the decrease of length and cross dimension of fibrin tissue, as well as increase of pore sizes; upon exposure to high-intensity US, the acceleration of medicamentous fibrinolysis is witnessed.
References
-
Lauer C. G., Burge R., Tang D. B., et al. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation, Vol. 86, No. 4, 1992, p. 1257-1264.
-
Behrens S., Daffertshofer M., Spiegel D., et al. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound. Med. Biol., Vol. 25, No. 2, 1999, p. 269-273.
-
Suchkova V. N., Baggs R. B., Francis C. W. Effect of 40-kHz ultrasound on acute thrombotic ichemia in a rabbit femoral artery thrombosis model. Enhancement of thrombolysis and improvement in a capillary muscle perfusion. Circulation, Vol. 101, No. 19, 2000, p. 2296-2301.
-
Tachibana K. Enhancement of fibrinolysis with ultrasound energy. J. Vasc. Interv. Radiol., Vol. 3, No. 2, 1992, p. 299-303.
-
Hong A. S., Chae J. S., Dubin S. B., et al. Ultrasonic clot disruption: an in vitro study. Am. Heart J., Vol. 120, No. 2, 1990, p. 418-420.
-
Trubestein G., Engele C., Etzel F., et al. Thrombolysis by ultrasound. Clin. Sci. Mol. Med., Vol. 3, 1976, p. 697-698.
-
Philippe F., Drobiski G., Bucherer C., et al. Effects of ultrasound energy on thrombi in vitro. Cath. Cardiovasc. Diagn., Vol. 28, No. 2, 1993, p. 173-178.
-
Adzerikho I. E., Mrochek A. G., Dmitriev V. V., et al. Ultrasound fibrin clot destruction in vitro in the presence of fibrinolytic agent. Ultrason. Sonochem., No. 8, 2001, p. 315-318.
-
Ernst A., Schenk E., Gracewski G., et al. Ability of high-intensity ultrasound to ablate human atherosclerotic plaques and minimize debris size. Am. J. Cardiol., Vol. 68, No. 2, 1991, p. 242-246.
-
Bielychier V. A., Varietskhaya T. V., Vieriemieienkho K. N., et al. Methods of Determination of Fybrynogen and Components of Fybrynogen of the Plazma of Human Blood: Methodical Recommendations. Kiev, 1983, 20 p., (in Russian).
-
Goldstein J., Newbern D., Etchlin P., et al. Raster Electronic Microscopy and X-ray Microanalysis. In two Volumes, Moscow, Mir, 1984, Vol. 1: 303 p., Vol. 2: 348 p., (in Russian).
-
Ghlanch S. Medical – Biological Statistics. Moscow, Practica Publishing House, 1999, 459 p., (in Russian).
-
Lakin G. F. Biometry. Moscow, Higher School Publishing House, 1990, 352 p., (in Russian).
-
Sabovic M., Lijnen H. R., Keber D., Collen D. Correlation between progressive absorption of plasminogen to blood clots and their sensitivity to lysis. Thromb. Haemost., Vol. 64, No. 3, 1990, p. 450-454.
-
Glover C. J., McIntire L. V., Leverett L. B., et al. Effect of shear stress on clot structure formation. Trans. Am. Soc. Artif. Int. Organs., Vol. 20, 1974, p. 463-468.
-
Sobel B. E., Nachowiak D. A., Fry E. T. A., et al. Paradoxical attenuation of fibrinolysis attributable to “plasminogen steal” and its implications for coronary thrombolysis. Coronary Artery Dis., Vol. 1, 1990, p. 111-119.
-
Carr M. E., Hardin C. L. Fibrin has larger pores when formed in the presence of erythrocytes. Am. J. Physiol., Vol. 253, No. 5, 1987, p. 1069-1073.
-
Rosenschein U., Frimerman A., Laniado S., et al. Study of the mechanism of ultrasound angioplasty from human thrombi and bovine aorta. Am. J. Cardiol., Vol. 74, No. 12, 1994, p. 1263-1266.
-
Anand S., Wu J. H., Diamond S. L. Enzyme mediated proteolysis of fibrous biopolimers: disolution front movement in fibrin or collagen under conditions of diffusion or connective transport. Biotechnol. Bioeng., Vol. 48, 1995, p. 89-107.
-
Nyborg W. L. Ultrasonic microstreaming and related phenоmena. Br. J. Cancer., Vol. 45, No. 5, 1982, p. 156-160.
-
Miller D. L., Thomas R. M., Williams A. R. Mechanisms for hemolysis by ultrasonic cavitation in the rotating expoure system. Ultrasound. Med. Biol., Vol. 17, No. 2, 1991, p. 171-178.
-
Everbach E. C., Francis C. W. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound. Med. Biol., Vol. 26, 2000, p. 1153-1160.
-
Kiseliov M. G., Minchenya V. T., Stepanenko D. A. Ultrasound in Medicine. Minsk, “Technical Literature” Publishing House, 2009, 428 p., (in Russian).
-
Miller D. L. Particle gathering and microstreming near ultrasonically activated gas-filled micropores. J. Acoust. Soc. Am., Vol. 84, No. 4, 1988, p. 1378-1387.
-
Alliger H. Ultrasonic disruption. Am. Laboratory, No. 7, 1975, p. 75-85.
About this article
This work has been supported by Research Council of Lithuania, Project TAP LB 18/2012 and Project Go-Smart, VP1-3.1-ŠMM-08-K-01-015.
