Abstract
Objectives of this study is to evaluate possibilities for early detection of udder’s diseases of Lithuanian Black and White cows and compare thermography and routine California mastitis test (CMT) results. Cows were examined in the negative and at positive ambient temperature. Infrared thermography (IRT) applied skin surface thermograms of teats in the area of Furstenberg’s rosette analyzed as posing evidence based signs of subclinical mastitis coincide with the CMT. It is shown, that subclinical inflammatory processes of cow’s mammary gland can be simply and successfully identified at an early stage of pathology development applying the thermal imaging method. IRT results measuring the skin surface temperature of teat sphincters of healthy dairy cows and cows with subclinical mastitis were completely similar to the CMT findings. Effectiveness of IRT does not depend from ambient conditions: inflammation can be easily detected both in positive and negative ambient temperatures. We confirm IRT being as alternative, noninvasive, high sensitive, rapid and portable method for subclinical various origins inflammatory processes of cow’s udder, which can be certified for practical subclinical mastitis screening and subsequent detection.
1. Introduction
Bovine mastitis is the most significant disease of dairy herds and has huge effects on farm economics due to marked reduction in qualitatively milk production and high treatment costs. European Union legislation (Regulation 853/2004) stresses that milk selected for human consumption must originate from healthy animals [1]. Worldwide it places a heavy economic burden on milk producers. The ideal means of dealing with mastitis is to prevent it from happening. However, despite the best prevention and control programs, mastitis will occur and the animal body generally defends itself against injury and infection. Detection of mastitis is generally based upon indicators of the inflammation of the mammary glands that mostly had local or generalized microbial infection origin [2]. When infection occurs leukocytes gather to engulf bacteria and stop the spread of infection. High leukocyte counts in milk strongly indicate mastitis-causing bacteria presence. These processes include increased leukocytes infiltration resulting in alterations in the chemistry of the milk. Subsequently it results hydrolysis of milk proteins by hydrolytic enzymes and oxidative substances released from phagocytes, alterations in milk pH and ionic solutes. Mastitis also is associated with an inflamed quarter together with a change in the appearance of the milk. These changes appear due to the effect of the cow’s inflammatory response to infection. On this understanding milk and milk products have been potential to transmit pathogenic organisms or its pathogenic products, such a toxin to humans, because milk of cows with subclinical mastitis have no visible differences, is accidentally mixed into bulk milk, and hereby enters food chain [3].
Technological advances including marked development of the proteomic and genomic investigation methods improve sensitivity of assays used for the mastitis detection [1]. It takes possibility to check the quality of the milk through detection of mammary gland inflammation and diagnosis of the infection and its causative pathogens [4]. Monitoring of udder health status and milk quality can be routinely determined by assessing high level of the somatic cell counts (SCC). Perfectly, uninfected mammary glands should have somatic cell counts no more than 50,000 cells/ml or less, but practically cell counts less than 200,000 cells/ml are taken as indicating the absence of infection. The dead and sloughed off mammary epithelial cells, in addition to the dead leukocytes, are secreted into the milk also resulting in high milk SCC [1]. Basing on this in veterinary practice as gold standard is commonly used California mastitis test (CMT), which indirectly measures the SCC in milk samples. The mechanism of CMT is simple-reagent is added to milk, reacts with a part of the leukocytes and forming a visible gel-like matrix substance. The more active infection presents the more number of leukocytes and proportionately more expressed gel. So, it is non-direct express indicator of mammary gland inflammation [5]. The advantage of the CMT is that it also assesses the level of infection of cows in the individual quarters. On another hand, limitation of CMT is comparatively high in price, what eliminate possibility perform it routinely for all animals during every day milking what would be request, but also for once a week. Disadvantages of CMT are what sometimes results can be difficult to interpret and method relatively has low sensitivity [1]. Important fact, what influence of a lot of other external factors like stress, some chronic inflammatory and infection diseases can also indirectly increase somatic cell counts. It shows CMT to be not so specific. Others described methods, including fossomatic SCC, electrical conductivity test (ECT), direct liquid chromatography-tandem assay are either not enough sensitive or expensive or complex to use. An increasing number of applications of new technologies to manage dairy herds initiate looking new technologies for early detection of inflammation as the main sign of mastitis and one of these methods could be IRT [6]. Detection of inflammation dates back to ancient Greece. Applying of IRT developed from 1953 and has numerous applications in human clinical practice as prognostic and in some cases diagnostic approach but it’s applying in veterinary medicine remain uncommon [7, 8]. Barth [9] suggested applying IRT for udder inflammation detection and practical use of this method for the early subclinical mastitis diagnostics. Several authors also applied IRT for diagnosing udder diseases and scanned the temperature on the teat tip, on the center, and on the teat base at the same time with the surface temperature of the udder base [10-12], but they made numerous experiments in positive environment temperature with different breed cows and possibly according to this reported enough contrast results. Lack of proper means motivates us to offer the new methodologies. The purpose of our study was to evaluate IRT efficacy and practical using as evidence based noninvasive method for early udder inflammation detection and its validation comparing with routine CMT results.
2. Methods and materials
The study was carried out from 2013 of November to 2014 of March on the Lithuanian Black and White healthy dairy cows. 57 cows were examined in the negative ambient temperature and 95 respectively at positive temperature. The cows were kept loose in cowsheds, in shallow pits, in cold barn, without bedding, being loose, with rubber mats for lying, trails made of grating floor with manure channels underneath. In the premises, the light/dark cycle was 12/12-h per day. Negative ambient temperature in average was –1±0.2 °C (typical winter period temperature of the year in the cowshed) at the relative humidity 35 % when the positive ambient temperature in average was +18±1.2 °C (typical summer period temperature of the year in the cowshed) the relative humidity 75 %. To exclude possible clinical disturbances, we simultaneously measured standard body temperature using a rectal thermometry which in all studied animals was in normal range.
The mammary gland health state was monitored by measuring the temperature of teats in the area of Furstenberg’s rosette sphincter before milking under the positive and negative ambient temperatures, what is typical for middle European region. Cows groups were formed according to the previous CMT diagnostic reactions: the milk was tested applying a CMT for selecting sub/clinical mastitis. Then temperature changes in each teat (608) of the udder were evaluated for sub-clinical mastitis testing by IRT. Basing on the study results and according to the positive/negative ambient temperature udder quarters were divided into 4 groups: group NS0 (control) – a negative CMT reaction and a negative ambient temperature; group TS0 (control) – at a positive ambient temperature and a negative CMT reaction; NS1 – a positive CMT reaction, determined the subclinical mastitis at a negative ambient temperature, and TS1 – a positive CMT reaction at a positive ambient temperature. Temperature indications of the teat skin surface of all groups of cows – NS0, NS1, TS0 and TS1 – were analyzed, and compared.
2.1. IRT methodology and device characteristics
For measuring the skin surface temperature of the udder area we used the high-sensitivity thermal camera “ThermaCAM P640” (FLIR Systems, USA). Diameter of 142 mm LCD (1024×600 pixels), a built-in external liquid-crystal display screen; thermal sensitivity (NETD) up to 0.06 °C at 30°C, detector FPA type, uncooled, 640×480 pixels, spectral range scanning frequency – up to 50 Hz. The range of the temperature measurement was selected between –40°C to +120°C, the viewing angle – 45°×34°. The distance from the test surface to the camera was from approximately 2 m, emissivity – 0.98. Thermal image was visually assessed using the color scale “Rainbow HC”. The thermograms initially were analyzed with “FLIR Tools 2.0 Pro” analysis program and later – by specially adopted program software.
2.2. CMT methodology for detection and assessment of subclinical mastitis
For routine detection of mastitis all animals were investigated before the main milking procedure: the small amounts of milk were conventionally examined applying the CMT diagnostic. In addition to surfactants, the test contains bromocresol purple, which indicates the milk pH by its altered color spectrum. The interaction of the surfactant (detergent) with the mastitis milk affects the nucleus of somatic cells and the viscosity of the mixture increases. The assessment of milk reaction applying express SCC diagnostic test procedure is provided in the Table 1.
Table 1The assessment of milk reaction applying SCC express diagnostic test
Points | Changes in the consistency of the mixture | Limits in SCC, thousand / cm3 | Average of SCC, thousand / cm3 |
1. | The consistency of the mixture is homogeneous, liquid, without visible changes (reaction is negative) | < 200 | 100 |
2. | Minor flakes appear, which vanish while turning the plate (trace) | 150-500 | 300 |
3. (+) | Minor clots are formed, the viscosity of the mixture increases (reaction is weakly positive) | 400-1500 | 900 |
4. (++) | The mixture is viscous, a clot is visible while turning the place and is localized in one place (the reaction is moderately positive) | 800-5000 | 2700 |
5. (+++) | A gummy, viscous mixture is formed, a clot of the form of an albumen is clearly seen and it drops out when pouring the mixture out of the plate (the reaction is strongly positive) | > 5000 | 8100 |
The reaction was considered negative (S0) when the changes in the consistency of the mixture constituted 1 or 2 points. The reaction was considered positive (S1) when the changes in the consistency of the mixture constituted 3, 4 and 5 points respectively.
2.3. Statistical analysis
The obtained statistical data were calculated employing SPSS software (version 15, SPSS Inc., Chicago, IL). The research data were processed applying statistical analysis method and using the statistical package “R 2.9.1.” (http://www.r-project.org) and WinExel program. Arithmetic means of samples (), standard square deviations (), coefficients of variation (), standard deviations () were calculated. The reliability of the difference of arithmetic means () was determined by applying the Student’s test [13]. The correlation () coefficients of the teat temperature and CMT results were calculated and their reliability at different ambient temperatures was determined. The analysis of variance (ANOVA) was applied to determine the influence of the ambient temperature on the teat temperature and CMT results. The results were considered reliable at 0.05.
3. Results and discussion
In recent years IRT has proven particularly useful both in the diagnostic and physiological assessments, highlighting its characteristic as noninvasive and non-contact technique. The primary advantages for using IRT in humans to aid in the detection of pathology include the fact that the method is absolutely painless, can be remotely operated, diagnostics can be made rapidly in real time, the technique is safe and the cost of the equipment is competitive with other diagnostic approaches [14, 15].
IRT has been successfully used and become popular in the prognosis and diagnosis of lot people health disturbances: diabetic neuropathy-angiopathy [16], vascular (mainly lower limb) disorders [17], breast or skin cancer detection, thermoregulation studies [18, 19] dermatology, diagnostic of rheumatologic diseases and bowel ischemia [20]. Nanni Costa et al. reported IRT not requiring any contact and is therefore being a completely non-invasive technique that allows recording measurements on subjects difficult to reach or to approach, or moving subjects [26].
Early subclinical mastitis detection became important practical problem by now. Redness due to vascularization or blood flow, pain (hypersensitivity), swelling, and hyperthermia are major signs already in the late stage of inflammation and infection. Gross changes in the milk such as the presence of flakes, clots or serous milk also can’t be routinely observed at the time of robotic milking. Regarding the physiological assessments, IRT has proven to be useful in detecting temperature variations of specific body parts of the cows in certain physical and environmental conditions. Analysis of surface of teats skin temperature using IRT may allow characterization of changing health status of udder. Subclinical infection of udder is enough difficult to detect due to the absence of any visible clinical symptoms. Thus it’s important to diagnose mastitis as early as possible to increase the opportunity for a favorable treatment outcome and reduce the losses associated with a reduction in the quality and quantity of milk [21]. Skin surface temperature reflects the underlying circulation and tissue metabolism [22], which is under the control of the sympathetic nervous system and noradrenergic sympathetic neurons in mammary gland [23]. These factors suggest that determination of skin surface temperature using IRT may evidence based assess mammary gland health status. These characteristics have a significant advantage in modern agriculture where large numbers of animals in intensively raised groups are common [24].
The findings obtained from our investigation markedly differ from reported findings in the several latest studies [10-12]. We are of the opinion that at first it’s therefore because of animals were investigated not from the identical view (angle) position, what can misrepresent thermographic image. Regions and point of interest for IRT investigation of the udder also differs. Secondly, quality and sensitivity of the IRT cameras also was different (mainly – more less) as well as thermogram analyzing software’s [6, 10, 11].
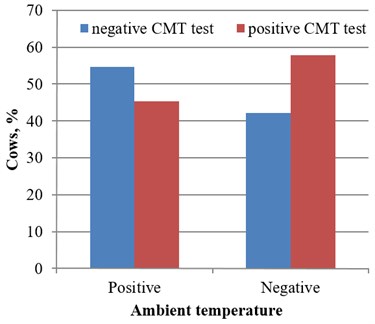
In 57 cows examined at the negative ambient temperature the routine CMT was positive for 33 (57.9 %), and negative for 24 (42.1 %) cases. Respectively, at positive ambient temperature, the CMT was positive for 43 (45.3 %) and negative for 52 dairy cows (54.7 %). Analysis and comparing temperature changes with CMT test results showed that 134 udder quarters of group NS0 (control) were without signs of subclinical mastitis at negative ambient temperature. 94 udder quarters with positive reaction were determined in group NS1 (CMT test reaction was positive). Respectively at positive environment temperature, 260 udder quarters (TS0 group) were without signs of subclinical mastitis and 120 udder quarters of cows (group TS1) reacted positively (Fig. 1).
Fig. 1CMT test results under the positive and negative ambient temperature
At the negative ambient temperature, the skin surface temperature of teat sphincters of group NS1 cows was on average 9.62 °C (43.5 %) higher (0.001) in comparison with group NS0 (control). At a positive environment temperature, the skin surface temperature of teat sphincters of group TS1 cows was in 4.60 °C (17.8 %) higher (0.001) in comparison with group TS0 (control).
Analysis of the surface temperature of the teat area of Furstenberg’s rosette demonstrated increasing temperature in 3.72 °C (16.8 %, 0.001) in group TS0 comparing with group NS0 at a positive environment temperature. Accordingly, the mean surface temperature of teats of group NS1 cows was in 1.31 °C (4.30 %) higher at negative ambient temperature than the mean temperature of group TS1 at positive ambient temperature (0.001), (Figs. 2-6).
Fig. 2Teat’s temperature dynamic on healthy and cows with positive CMT at different ambient temperature
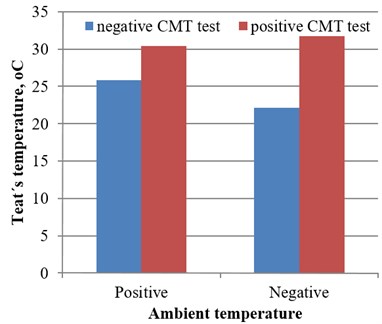
Fig. 3Example of subclinical mastitis of right rear and right front udder quarter, marked by arrows (CMT – negative)
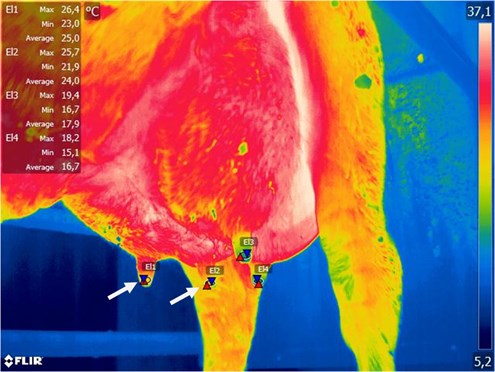
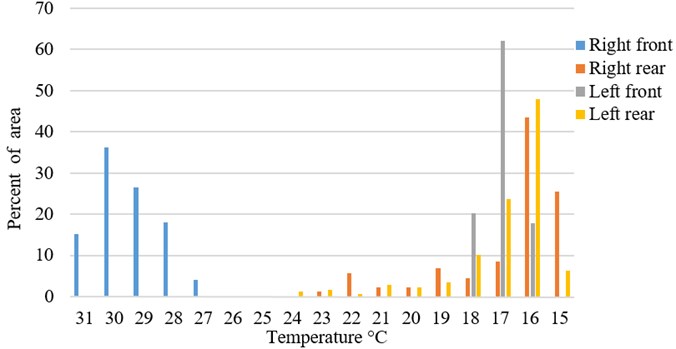
Fig. 4Typical temperature distribution in percentage of measured area (Fig. 3 sample)
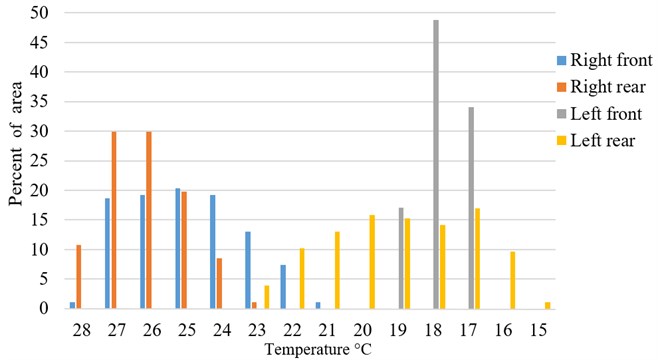
Fig. 5Typical example of inflamed right front udder quarter, marked by arrow (temperature difference – about 15 °C, CMT test – positive)
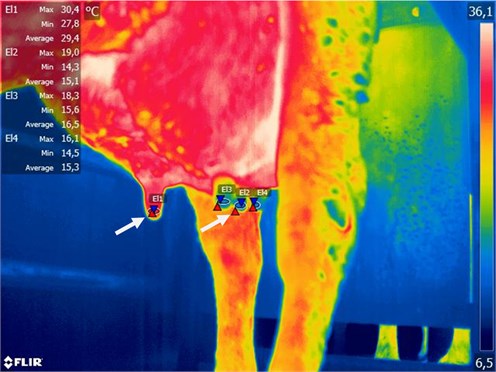
Fig. 6Typical temperature distribution in percentage of measured area (Fig. 5 sample)
Vegricht [25] noticed that thermography can become an indicator of increased average temperature of the teat tips after milking. He founded that the average temperature of the teat tip increased by 1.7 °C-2.7 °C as compared to the temperature before milking. In our cases we avoid possible mistakes, associated with robotic milking procedures influence to the teats surface temperature. First of all, we measured temperature before milking. Second, temperature differences indicating possible inflammation were higher: 9. 62 and 4.6 °C at negative and positive ambient temperatures respectively, which is on the outside of normal physiological teats temperature changes. So, using IRT takes possibility to look to the problem of mastitis screening and detection by new angle. In our study we determined a strong positive correlation of the increased teat temperature and positive CMT results between groups NS0 and NS1 at the negative ambient temperature ( 0.769; 0.01). As we expected the temperature differences in this cases were more than obvious. We were surprised getting the same strong positive correlation of the teat area of Furstenberg’s rosette temperature and CMT results between groups TS0 and TS1 determined at the positive environment ( 0.717; 0.01). In cases of mastitis, udder surface temperature as measured by IRT has shown also a strong correlation ( 0.92) with the CMT test.
We also determined that in groups NS0 and TS0 the ambient temperature influenced the skin surface temperature of the teat sphincters and this influence was however statistically significant (0.01). Combined procedures including new technologies e. c. using high sensitivity IRT and CMT can serious improve early diagnostic of subclinical mastitis independently from ambient temperature.
Temperature contour map and temperature gradient map (Fig. 7) provides a deeper insight into temperature distribution. An area given in this map corresponds to a zoomed udder area in Fig. 3 and Fig. 5 respectively. In order to calculate gradient field initial images were converted from RGB map to temperature map. Gaussian smoothing was applied to temperature map:
where is the distance from the origin in the horizontal axis, is the distance from the origin in the vertical axis, and is the standard deviation of the Gaussian distribution. In practice we need to calculate matrix convolution to apply Gaussian smoothing, thus as we have discrete data, the discrete Fourier transform was used, which is the primary tool of digital signal processing. The DFT transforms time or space-based data into frequency based data. The 2D discrete transform is defined as follows:
where and are the discrete spacial frequencies, and are the number of samples in and directions in spacial and frequency domains, and is the 2D discrete spectrum of . The inverse of 2D discrete transform is defined as follows:
A direct calculation of -point DFT requires complex multiplications and complex additions. To reduce the computation speed, various FFT (Fast Fourier Transform) algorithms has been developed to efficiently compute the discrete Fourier transform and its inverse.
The gradient map of temperatures , was calculated:
A collection of vectors pointing in the direction of increasing temperature was retrieved. A graphical representation temperature gradient field together with contour lines is given in Fig. 7.
Fig. 7Temperature contour map and temperature gradient map corresponding to a) Fig. 3 and b) Fig. 5
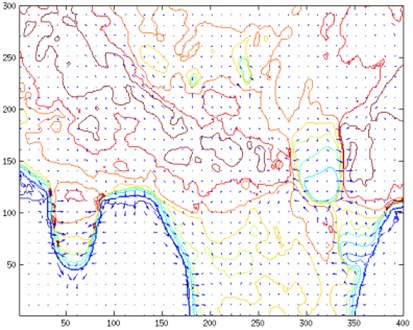
a)
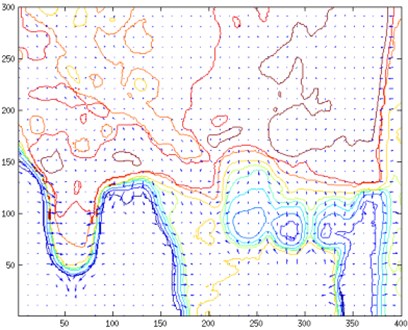
b)
We agree that improved technological advances using immunology-based assays, such as ELISA, biosensors, proteomic and genomic origin inflammation-related biomarkers markers can provide reliable information and be used to detect inflammation-related biomarkers present in the milk at different stages of subclinical mastitis, but all of them required serious competitive validation. Other currently used “on line” mastitis detection tests are either not enough sensitive for conclusive diagnosis and real udder health status formation or including multiplex or “real-time” PCR are capable detect mastitis-caused pathogens, what can’t be associated with real mastitis in particular situation. On another hand all this novels assays had more or less limitations, are mostly too expensive and for the meantime is surreal from practical routine application.
Thus, IRT alone can be enough informative and sensitive approach, which can be certified for practical apropos subclinical mastitis screening and subsequent detection. We also consider method could be successfully automatized and adopted for more wide users’ spectrum.
4. Conclusions
IRT is able to discover changes that have not yet caused clinical signs in apparently healthy subjects. IRT results measuring the skin surface temperature of teat sphincters of healthy dairy cows and cows with subclinical mastitis were very similar to the CMT findings. Subclinical inflammatory processes can be identified at an early stage “on-site” and “on-line” applying the method of thermal imaging. We expect IRT being enough sensitive, simple, portable, rapid and user friendly method for screening and detection of early stage inflammation of bovine mammary gland.
References
-
Viguier C., Arora S., Gilmartin N., Welbeck K., O’kennedy R. Mastitis detection: current trends and future perspectives. Trends in Biotechnology, Vol. 27, Issue 8, 2009, p. 486-493.
-
Klimienė I., Ružauskas M., Špakauskas V., Mockeliūnas R., Pereckiene A., Butrimaitė-Ambrozevičienė Č. Prevalence of gram positive bacteria in cow mastitis and their susceptibility to beta-Lactam antibiotics. Veterinarija ir Zootechnika – Veterinary Medicine and Zootechnics, Vol. 56, Issue 78, 2011, p. 65-72.
-
Zecconi A., Hahn G. Staphylococcus aureus in raw milk and human health risk. Bulletin of the International Dairy Federation, Vol. 345, 2000, p. 15-18.
-
Pyorala S. Indicators of inflammation in the diagnosis of mastitis. Veterinary Research, Vol. 34, 2000, p. 565-578.
-
Schalm O. W., Noorlander D. O. Experiments and observations leading to development of the California mastitis test. Journal of the American Veterinary Medical Association, Vol. 130, 1957, p. 199-204.
-
Polat B., Colak A., Cengiz M., Yanmaz L. E., Bastan A., Kaya S., Hayirli A. Sensitivity and specificity of infrared thermography in detection of subclinical mastitis in dairy cows. Journal of Dairy Science, Vol. 93, Issue 8, 2010, p. 3525-3532.
-
Embaby S., Shamaa A. A., Gohar H. M. Clinical assessment of thermography as a diagnostic and prognostic tool in horse practice. Proceedings of Inflammation, Orlando, USA, 2002, p. 30-36.
-
Markel A. L., Vainer B. Infrared thermography in diagnosis of breast cancer (review of foreign literature). Terapevticheskii Arkhiv, Vol. 77, 2005, p. 57-61.
-
Barth K. Basic investigations to evaluate a highly sensitive infrared-thermographtechnique to detect udder inflammation in cows. Milchwissenschaft Milk Science International, Vol. 55, Issue 11, 2000, p. 607-609.
-
Colak A., Polat B., Okumus Z., Kaya M., Yanmaz L. E., Hayirli A. Early detection of mastitis using infrared thermography in dairy cows. Journal of Dairy Science, Vol. 91, Issue 11, 2008, p. 4244-4248.
-
Hovinen M., Siivonen J., Taponen S., Hanninen M., Pastell M., Aisla A. M., Ptorela S. Detection of clinical mastitis with the help of a thermal camera. Journal of Dairy Science, Vol. 91, Issue 12, 2008, p. 4592-4598.
-
Franze U., Geidel S., Heyde U., Schroth A., Wirthgen T., Zipser S. Investigation of infrared thermography for automatic health monitoring in dairy cows. Zuchtungskunde, Vol. 84, Issue 2, 2012, p. 158-170.
-
Juozaitienė V., Kerzienė S. Biometrics and Computer Data Analysis. Kaunas, 2001, p. 115, (in Lithuanian).
-
Jiang L. J., Ng E. Y., Wu S., Pan F., Yau W. Y., Chen J. H., Yang Y. Aperspective on medical infrared imaging. Journal of Medical Engineering and Technology, Vol. 29, Issue 6, 2005, p. 257-267.
-
Lahiri B. B., Bagavathiappan S., Jayakumar T., Philip J. Medical applications of infrared thermography. A review. Infrared Physics and Technology, Vol. 55, Issue 4, 2012, p. 221-235.
-
Ring F. Thermal imaging today and its relevance to diabetes. Journal of Diabetes Science and Technology, Vol. 4, Issue 4, 2010, p. 857-862.
-
Bagavathiappan S., Saravanan T., Philip J., Jayakumar T., Raj B., Karunanithi R., Panicker T. M., Korath P., Jagadeesan K. Investigation of peripheral vascular disorders using thermal imaging. The British Journal of Diabetes and Vascular Disease, Vol. 8, 2008, p. 102-104.
-
Ludwig N., Gargano M., Formenti D., Bruno, Ongaro L., Alberti G. Breathing training characterization by thermal imaging: a case study. Acta of Bioengineering and Biomechanics, Vol. 14, Issue 3, 2012, p. 42-47.
-
Formenti D., Ludwig N., Gargano M., Gondola M., Dellerma N., Caumo A., Alberti G. Thermal imaging of exercise-associated skin temperature changes in trained and untrained female subjects. Annals of Biomedical Engineering, Vol. 41, Issue 4, 2013, p. 863-71.
-
Brooks J. P., Perry W. B., Putnam A. T., Karulf R. E. Thermal imaging in the detection of bowel ischemia. Diseases of the Colon and Rectum, Vol. 43, Issue 9, 2000, p. 1319-1321.
-
Gianneechini R., Rivero R., Delucci I., Moreno Lopez J. Occurrence of clinical and sub-clinical mastitis in dairy herds in the West Littoral Region in Uruguay. Acta Veterinaria Scandinavica, Vol. 43, Issue 4, 2002, p. 221-230.
-
Berry R. J., Kennedy A. D., Scott S. L., Kyle B. L., Schaefer A. L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: potential for mastitis detection. Canadian Journal of Animal Science, Vol. 4, 2003, p. 687-693.
-
Paulrud C. O., Clausen S., Andersen P. E., Rasmussen M. D. Infrared termography and ultrasonography to indirectly monitor the influence of liner type and overmilking on teat tissue recovery. Acta Veterinaria Scandinavica, Vol. 46, 2005, p. 137-147.
-
Schaefer A. L., Cook N. J. Heat Generation and the Role of Infrared Thermography in Pathological Conditions. Thermography: Current Status and Advances in Livestock Animals and in Veterinary Medicine, Fondazione Iniziative Zooprofilattiche E Zootecniche, Brescia, Italy, 2013, p. 69-78.
-
Vegricht J., Machálek A., Ambrož P., Brehme U., Rose S. Milking-related changes of teat temperature caused by various milking machines. Research in Agricultural Engineering, Vol. 53, Issue 4, 2007, p. 121-125.
-
Nanni Costa L., Magnani D., Cafazzo S., Luzi F., Redaelli V., Dall’olio S. Relationships between the quality of thermographic image and the measure of skin temperature on piglets. Acta Argiculturae Slovenica, Vol. 4, 2013, p. 127-130.
About this article
Ina Pampariene performed and analyzed CMT tests. Judita Zymantiene coordinated research procedures. Rasa Zelvyte performed manual testing for subclinical mastitis and excluding animals with other types of mammary gland inflammation. Vaidas Oberauskas performed data statistical analysis. Vincentas Veikutis performed thermography procedures and testing. Arunas Stankevicius was responsible for control of microbial antigens in cow’s udder by using somatic cell counts and molecular methods. Dalia Marciulionyte was responsible for literature analysis and microbiological control of selected animals. Paulius Palevicius performed numerical analysis of thermographic images to retrieve contour and gradient maps of cow udder temperatures.
